
by Scarlet Faro, 2nd year student, MPhil Textile Conservation.
In my second year on the MPhil Textile Conservation, one of the objects I worked on was a hand-made cotton cutwork lace jabot, part of the collection at Dumfries Museum and Camera Obscura. A jabot is a decorative neckwear accessory, worn to conceal the front opening of a shirt or blouse.[1] At first, the object appeared fairly uncomplicated, but on closer study I realised it would in fact require a more complex treatment, including conservation bleaching. As this is not a commonly used practice, I thought I would share my experience.
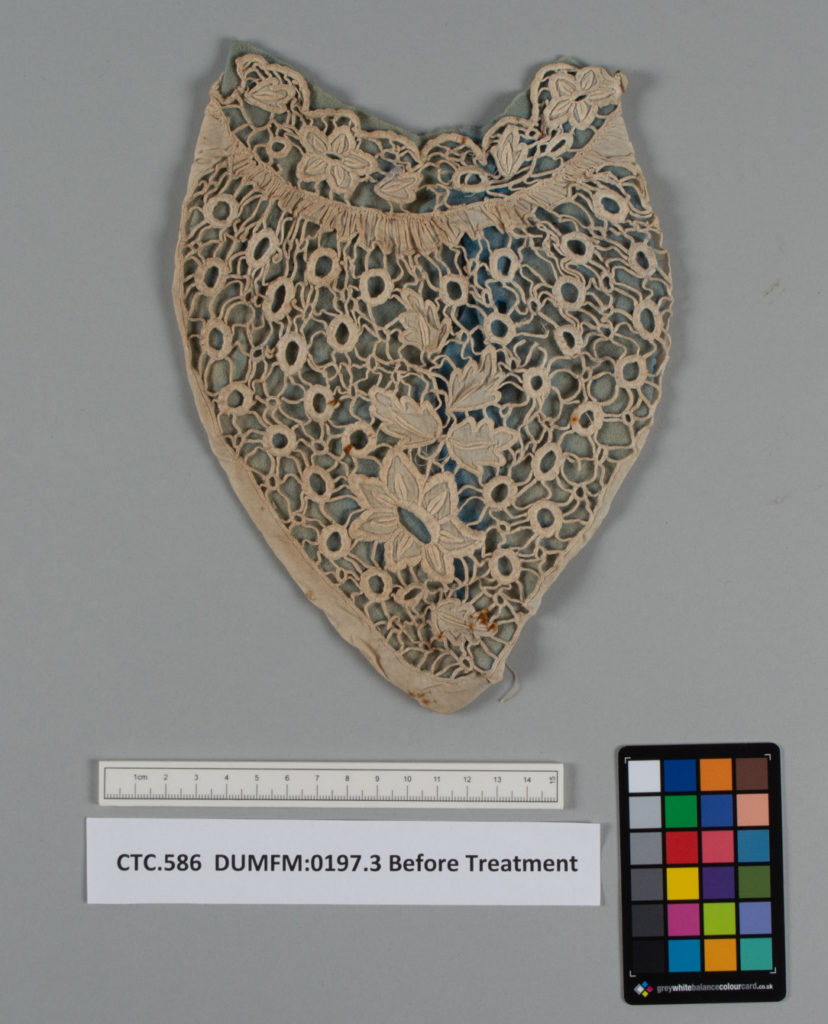
Fig 1 & 2- DUMFM:0197.3 Jabot before treatment, front (left) and back (right). Images © University of Glasgow and courtesy of Dumfries Museum and Camera Obscura. 2023
There were several dark orange-brown stains on the front of the cutwork lace layer of the jabot, characteristic signs of rust. To confirm this, I tested the stains for the presence of iron (II) ions using indicator test strips (Bathophenanthroline Indicator Paper), which provided a positive result.[2] The presence of rust on textile fibres can lead to weakening and further damage if left untreated.
In order to facilitate the treatment, the two layers of the jabot were first separated and each element was wet cleaned. As expected wet cleaning the cutwork lace layer did not remove the orange-brown stains, and I subsequently decided, in consultation with the client, to carry out a localised conservation bleaching treatment with the use of a reducing agent. The reducing agent in this case converts the insoluble iron (III) ions to soluble iron (II) ions. The reason conservation bleaching is rarely used by textile conservators is that it can potentially damage delicate textiles so it is only used where it is considered beneficial for the object and important for its role[3]. Neither I nor my classmates had seen a bleaching treatment carried out before, so I relied on source material and the help of my lecturers for advice, and I approached it with great care and lots of testing!
I carried out a series of tests with both sodium borohydride and sodium dithionite on rust-stained sample fabric (Figure 3). Sodium dithionite proved to be the most effective in reducing the stains. Different methods of application were trialled, including a fine brush and pipette. Brush application achieved the most success as this was easy to control and prevented too much wicking. Further control was achieved by undertaking the treatment on a vacuum suction table.

Once I had completed the testing stage, I was ready to begin the treatment on the cutwork lace layer. I initially started with a 2% concentration of sodium dithionite and deionised water (as indicated in the literature[4]), which I lightly applied onto each rust stain with a fine brush. Continually evaluating the process, it became clear that there was only a small reduction in colour, so I increased the concentration to 5%, the purpose of which was to achieve higher and faster efficacy while requiring less mechanical action, therefore less fibre damage. The 5% concentration immediately showed better results with many of the stains being considerably or completely reduced. Deionised water was brushed onto each stain after application, to rinse out the reducing agent solution and to help prevent it from wicking into non- stained areas and potentially creating lighter rings or halos. After completing the treatment, I soaked the object in deionised water for two 10-minute baths, to ensure all the reducing agent was completely flushed out.
As the 5% solution was proving to be a success, I was tempted to continue working on the stains. However, I noticed some fibres appeared to be weakening so I took the decision to stop the process at this point, having realised this was the stage at which I had achieved the optimum result. Overall, I was pleased with the outcome and grateful for the opportunity to practice a complicated treatment and learn some valuable lessons in the process.
Figures 4 and 5: Rust stains before (above) and after (below) application of reducing agent. Image © University of Glasgow and courtesy of Dumfries Museum and Camera Obscura. 2023
[1] John Peacock, The Chronicle of Western Costume, (London: Thames and Hudson, 1991), 218.
[2] Preservation Equipment Ltd, “Iron Gall Ink Test Paper,” PEL, Accessed Mar. 7, 2023, https://www.preservationequipment.com/Catalogue/Instruments/Other-Measuring-Equipment-Materials/Iron-Gall-Ink-Test-Paper-75mm-x-10mm-100-Strips-P539-3000.
[3] Salem, Rana T. A., Karen Thompson, and Mahesh Uttamlal. “Bleaching Cotton in Textile Conservation: A Closer Look Using Atomic Force Microscopy.” Heritage Science 10, no. 1 (2022/12/05 2022): 195.
[4] AIC Wiki, “BPG Bleaching: Treatment Variations,” May 5, 2022, Accessed Mar. 7, 2023, https://www.conservation-wiki.com/wiki/BPG_Bleaching



